Secondary Screening & Mechanism-of-Action
The Importance of Direct Binding Determination in Pharmacology
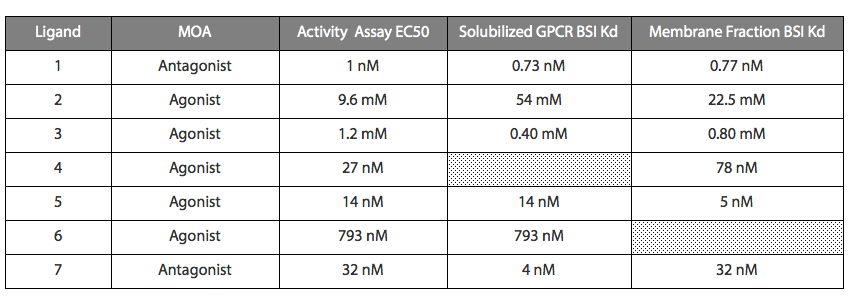
It is critical in any modern drug development effort to demonstrate that a pharmaceutical compound engages with its target. Confirmation of direct binding of candidate small molecules from an HTS screen to the target of interest is critical to verification of a suspected mechanism of action (MOA) for that compound. Establishing clear MOA is crucial not only to successful drug development efforts, but is increasingly demanded by regulatory entities such as the FDA and international equivalents. Additionally, determining the individual affinity of candidate small molecules serves a vital role in shaping further medicinal chemistry efforts by providing SAR information. The BSI platform provides this information, constituting a significant value proposition to the drug development process.
BSI allows direct binding measurements of libraries of small molecule compounds without the need for labeling. In this role, BSI serves as an efficient secondary screening platform and complement to HTS assays. Established platforms that rely on measurement of binding competition with radiolabeled or fluorescent compounds provide only an indirect assessment of affinity and can be highly probe dependent.
Both cell-based and cell-free functional activity assays provide invaluable data for screening and characterization of drug candidates. These types of assays are particularly useful in identifying putative ligands for targets such as GPCRs. However, these assays have some “black box” aspects in that binding and signal generation are several steps removed and can be affected by functional characteristics of many biomolecular interactions. Confirmation of agonism/antagonism and allosteric potentiation/inhibition from functional assays, and further, quantitative information on allosteric compound binding in a minimal system is a strength of BSI technology that cannot be supplied by functional assays alone. As integral membrane drug development projects gain prominence in pharmaceutical pipelines, label-free assay technologies such as BSI are poised to make a significant impact.
Please send us an email or give us a call at (615) 938-7050 in North America or +49 (171) 760-4450 in Europe
to find out how MSI can help accelerate your assay development and screening efforts.
APPLICATIONS
ON-DEMAND WEBINARS

 Review of Recent Publications Featuring Binding Data Produced by MSIBy MSI on January 26, 2016
Review of Recent Publications Featuring Binding Data Produced by MSIBy MSI on January 26, 2016 Conformation-Sensitive Assay Detection for Accelerated Drug Discovery ResearchBy MSI on February 10, 2015
Conformation-Sensitive Assay Detection for Accelerated Drug Discovery ResearchBy MSI on February 10, 2015
 Using BSI to Investigate Positive Allosteric Modulators of the M4 ReceptorBy MSI on September 3, 2014
Using BSI to Investigate Positive Allosteric Modulators of the M4 ReceptorBy MSI on September 3, 2014